
Glycerol is known as the eight major industrial raw materials. In the oil chemical production, glycerol is also the main source of economic benefits, so it has always been the concern of operators. From the point of view of recovering glycerol, it is hoped that the glycerol can be separated as much as possible in the oil chemical engineering, and the concentration of the separated glycerol should be as high as possible to reduce the energy consumption and production cost in refining. The recycling methods described below are carried out around these purposes.
Oils and fats can be hydrogenated directly to prepare fatty alcohols and glycerol. However, glycerol, the by-product of direct hydrogenation of oils and fats, is produced under hydrogenation conditions. Glycerol is prone to dehydration and produces a mixture of 1,3-propanediol and 1,2-propanediol. The existence of propylene glycol not only makes the separation difficult but also affects the quality of glycerol, so it is generally used to make fatty acid lipids and glycerol by lipid exchange reaction, and then hydrogenate fatty acid lipids to get fatty alcohol. At present, the proportion of fatty acid and glycerol produced by ammonolysis of fat to fatty amine and enzymatic hydrolysis of fat is very small in China, so this paper mainly discusses the method of recovering glycerol from waste saponification liquor of oil, sweet water of oil cracking and sweet water of oil alcoholysis.
Recovery of glycerol from soap and saponified waste liquor by saponification is one of the traditional oils and fats. The oil saponification is divided into batch type (Chinese name is big pot boiled soap) and continuous type. Soap boiling in large pot is the reaction of oil and caustic soda to form soap and glycerol under the action of direct steam. In order to improve the recovery of glycerol and the concentration of glycerol in saponified waste liquor, saponification, salting-out, washing, alkaline-out and finishing are often used.
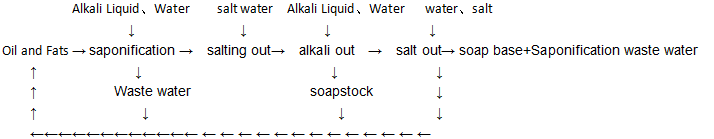
The saponification process requires that a certain amount of free alkali (0.3% – 0.5%) should be maintained in the whole process, and the saponification reaction should be carried out in a homogeneous state with a yield of about 95%.
The purpose of salting out is to separate glycerol from soap glue. The effect of salting out is to make the water inside the soap glue diffuse outward and make the soap particles condense and condense out of the brine because of the same ion benefit and osmotic pressure change after adding salt into the soap glue. This salting out water is used as saponification waste liquid to recycle glycerol. The amount of waste liquid is controlled by 1.1-1.5 times of the amount of oil.
The components of waste liquid water are: glycerol 6% – 8%; NaOH 0.1% – 0.5%; NaCI 10% – 15%; fat 0.1% – 1%; other colors, proteins, colloids and so on.
The purpose of alkaline precipitation is to make the oil saponified completely and crack the glycerol components in the oil as far as possible, so as to reduce the content of glycerol in the saponification base. The alkaline precipitation water can be returned to the saponification process to increase the content of glycerol in the saponification waste liquid.
The purpose of finishing operation is to adjust the moisture and electrolyte content of the soap base to meet the needs of the post-processing of the soap base.
The glycerol content in the hydrolyzed sweet water and saponified waste liquid has great influence on the processing cost of glycerol production. For example, when concentrated crude glycerol is used to produce crude glycerin, the consumption of steam will be greatly affected. Table 3.2 shows the theoretical value of the amount of water to be evaporated in the production of 1 t (80%) crude glycerol from saponified waste liquor or sweet water with different glycerol content. Table 3.3 is the percentage of energy saving in the saponification waste or sweet water.
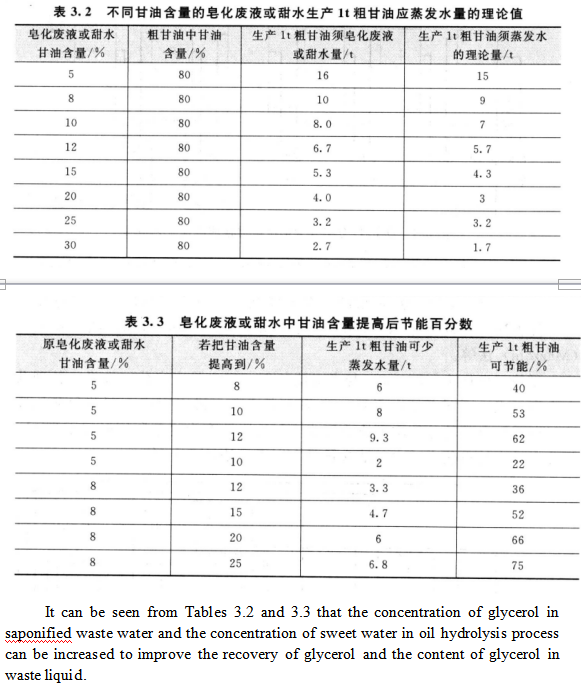
It can be seen from Tables 3.2 and 3.3 that the concentration of glycerol in saponified waste water and the concentration of sweet water in oil hydrolysis process can be increased to improve the recovery of glycerol and the content of glycerol in waste liquid.