Ethanol is one type of alcohol that has many properties quite similar to those of gasoline. These similarities make ethanol a highly attractive fuel for use as a gasoline substitute or as an alternative fuel for blending. The densities of ethanol and gasoline are almost identical although the energy content of ethanol is about 30% lower. On the other hand, since ethanol contains molecular oxygen that promotes more complete combustion, the difference in energy content does not become a major concern at low level of alcohol blends in gasoline.
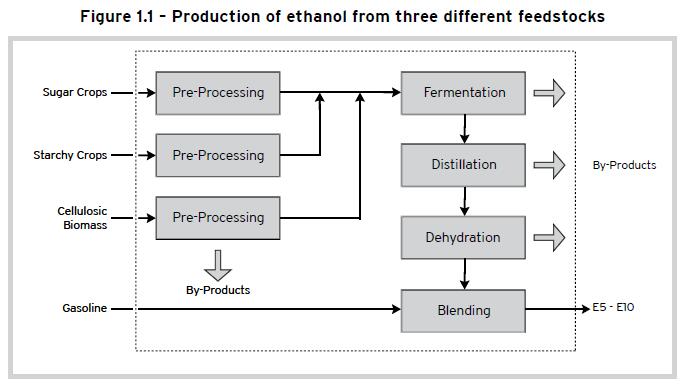
Ethanol can be produced by the fermentation of carbohydrates from various feedstocks. The feedstocks fall under three main categories: (a) sugarbearing feedstocks such as sugar cane; (b) starchy feedstocks such as cassava or corn; and (c) cellulosic feedstocks such as wood and agricultural residues such as bagasse. The production technologies for the first two types of feedstocks are fully mature and well-developed and are commercially available from various suppliers. However, the processing of cellulosic materials into ethanol using either acid or enzymatic hydrolysis is not yet fully developed commercially but is rapidly becoming technically and economically viable. Figure 1.1 shows schematically a simplified flow diagram for the production of ethanol from these three different feedstocks. Depending on the type and nature of feedstock, the pre-processing steps or operations may differ but there are basically three processes that are common for all three types of feedstocks: (a) fermentation of the sugars into ethanol; (b) distillation to separate the aqueous ethanol (95%) from the fermented mash; and (c) dehydration to produce anhydrous ethanol (>99.5%) suitable for blending with gasoline. In the case of starchy and cellulosic feedstocks, pretreatment through saccharification or hydrolysis is required in order to convert them to sugars that can be fermented by yeast into ethanol.
Fermentation is a series of chemical reactions that convert sugars to ethanol in the presence of suitable strain of yeast, which feed on the sugars. Ethanol and carbon dioxide are produced as the sugar (glucose) is consumed. The simplified fermentation reaction for a 6-carbon sugar, glucose, is as follows:
C6H12O6 = 2 CH3CH2OH + 2 CO2
The ethanol concentration in the fermented mash can attain a level of only 4% to 10% depending on the operating conditions and the strain of yeast being used. Ethanol is therefore recovered through distillation but only hydrous ethanol of about 95-96% can be produced through steam distillation of the fermented mash due to the formation of water-ethanol azeotrope. The product ethanol is withdrawn from the top of the distillation column while the spent fermented mash called distillery slops is withdrawn from the bottom and sent for further treatment before final disposal or reuse2. To make ethanol fully miscible with gasoline, it is necessary to further remove the residual water to produce anhydrous ethanol with a concentration of at least 99.5%. To attain this concentration, the hydrous ethanol has to undergo a suitable dehydration process or operation.
There are at least three widely-used dehydration processes to further remove water from the azeotropic ethanol-water solution. The first process that was used in many of the earlier ethanol plants is the so-called azeotropic distillation or ternary distillation process (as opposed to a binary or twocomponent distillation process). It consists of introducing a third component, benzene or cyclohexane, to the azeotropic solution which forms a heterogeneous azeotropic mixture in vapor-liquid-liquid equilibrium. When this mixture is distilled, anhydrous ethanol is produced at the bottom of the distillation column. Another early method is called extractive distillation, which consists of adding a ternary component that increases the relative volatility of ethanol. In this case, anhydrous alcohol is produced and withdrawn at the top of the distillation column.
Because distillation processes are energy intensive, a third method has been developed and accepted in majority of modern ethanol plants. This process uses molecular sieves to remove water from ethanol. In this process, ethanol vapor under pressure passes through a bed of molecular sieve beads. The pores of the beads are such that they allow the absorption of water but not ethanol. Two beds are normally used in parallel to allow the regeneration of one bed while the other is in use. This dehydration technology can save significant amounts of energy (up to 840 kJ/l) compared to conventional azeotropic or extractive distillation processes.
For the production of ethanol from corn, there are two main processes in commercial use: dry milling and wet milling. In the dry milling process the entire corn kernel is ground into flour, which is referred to as “meal.” The meal is then made into slurry by adding water. Enzymes are added to the mash to convert starch to dextrose, which is a simple sugar. Ammonia is added to control the pH and to provide nutrient for the yeast, which is added later. The mixture is processed at high temperatures to reduce the bacteria levels and transferred and cooled in fermentation tanks. This is where the yeast is added and conversion from sugar to ethanol and carbon dioxide takes place.
The entire process takes between 40 to 50 hours, during which time the fermenting mash is kept cool and agitated in order to facilitate yeast activity. After the process is complete, everything is transferred to distillation columns where the ethanol is removed from the stillage. The ethanol is dehydrated to about 99.5% using a molecular sieve system. A denaturant such as gasoline is added to the anhydrous ethanol to render the product not suitable for drinking. The remaining stillage undergoes a series of processes to produce feed for livestock. The carbon dioxide released from the process is collected and processed to produce industrial or food grade product.
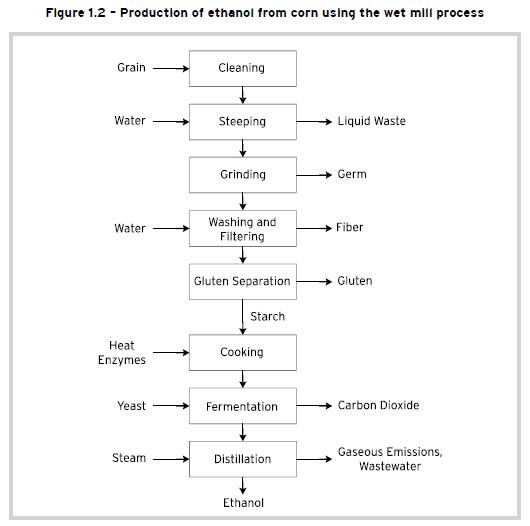
The process of wet milling takes the corn grain and steeps it in a dilute combination of sulfuric acid and water for 24 to 48 hours in order to separate the grain into many components. The slurry mix then goes through a series of grinders to separate out the corn germ. Corn oil is a by-product of this process and is extracted and sold. The remaining components of fiber, gluten and starch are segregated out using screen, hydroclonic and centrifugal separators.
The gluten protein is dried and filtered to make a corn gluten meals coproduct, which is used as a feed ingredient for poultry broilers. The steeping liquor produced is concentrated and dried with the fiber and sold as corn gluten feed to the livestock industry. The heavy steep water is also sold as a feed ingredient. The starch and remaining water can then be processed one of three ways: (a) fermented into ethanol through a similar process as dry milling; (b) dried and sold as modified corn starch; or (c) made into corn syrup. This process is shown schematically in Figure 1.2. The production of ethanol from corn using the wet mill process has become the technology of choice since it provides more product diversity and flexibility.
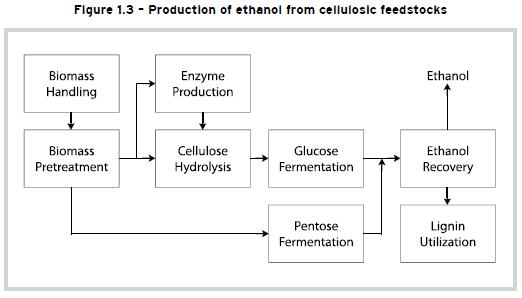
To produce ethanol from cellulosic feedstocks, several pre-treatment steps are necessary to convert cellulose into simple sugars that can be converted into alcohol using the conventional yeast fermentation process. This is shown schematically in Figure 1.3.
The first step is mechanical preparation through size reduction to make the material easier to handle and more susceptible to the subsequent pre-treatment steps. For example, agricultural residues go through a grinding process and wood goes through a chipping process to produce smaller and more uniform particle size. This is followed by acid pre-treatment. In this step, the hemicellulose fraction of the biomass is broken down into simple sugars. A chemical reaction called hydrolysis occurs when dilute sulfuric acid is mixed with the biomass feedstock. In this hydrolysis reaction, the complex chains of sugars that make up the hemicellulose are broken, releasing simple sugars. The complex hemicellulose sugars are converted to a mix of soluble five-carbon sugars, xylose and arabinose, and soluble six-carbon sugars, mannose and galactose. A small portion of the cellulose is also converted to glucose.
The next step is cellulose hydrolysis. In this step, the remaining cellulose is hydrolyzed to glucose. In this enzymatic hydrolysis reaction, cellulase enzymes are used to break the chains of sugars that make up the cellulose, releasing glucose. Cellulose hydrolysis is also called cellulose saccharification because it produces sugars. The cellulase enzymes needed for this process may be produced in-house in a separate enzyme production facility or may be purchased from outside suppliers. The glucose is converted to ethanol through yeast fermentation, as discussed earlier. The yeast feeds on the sugars and as the sugars are consumed, ethanol and carbon dioxide are produced.
The hemicellulose fraction of biomass is rich in five-carbon sugars, which are also called pentoses. Xylose is the most prevalent pentose released by the hemicellulose hydrolysis reaction. As glucose is converted to ethanol by yeast fermentation similar to those used in the first two types of feedstocks, the pentose (mainly xylose) is subjected to a different type of fermentation. In pentose fermentation, Zymomonas mobilis or other genetically engineered bacteria are used instead of yeast. Hydrous ethanol is recovered from the fermented mash through distillation and anhydrous ethanol is produced after dehydration, as in the previous processes described earlier.
Lignin and other byproducts of the biomass-to-ethanol process can be used to produce the electricity required for the ethanol production process. Burning lignin actually creates more energy than needed and selling electricity to outside users improves the economic viability of the process.